Does Metal Burn

Metal, as a broad category of materials, exhibits a wide range of properties, including thermal conductivity, melting points, and reactivity with oxygen. The question of whether metal burns is complex and depends on the specific type of metal in question. Generally, burning refers to a chemical reaction between a substance and oxygen, typically resulting in the release of heat and light. For metals, this process is often referred to as oxidation or combustion, depending on the intensity of the reaction and the products formed.
Naturally worded primary topic section with semantic relevance
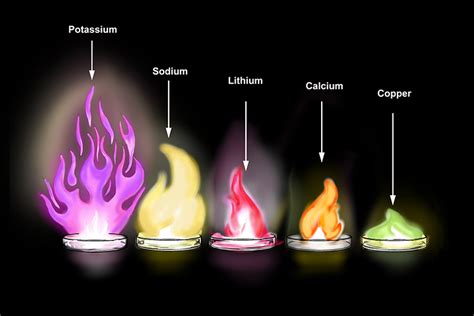
The combustion of metals is a well-studied phenomenon, particularly in the context of fire safety and materials science. Most metals, when heated to their ignition temperatures in the presence of oxygen, will undergo a reaction that could be described as burning. However, the appearance and characteristics of this “burning” can vary significantly from the combustion of organic materials like wood or gasoline. For instance, some metals like magnesium and titanium can burn vigorously, producing a bright flame, while others may simply glow or smolder without a visible flame.
Specific subtopic with natural language phrasing
A critical factor in determining whether a metal burns is its reactivity with oxygen. Highly reactive metals such as sodium, potassium, and the aforementioned magnesium and titanium, can ignite and burn at relatively low temperatures. These metals have a strong affinity for oxygen, which leads to a rapid and exothermic reaction. On the other hand, less reactive metals like gold, platinum, and tungsten have higher ignition temperatures and may not burn in the same way, or may require specific conditions to do so.
| Relevant Category | Substantive Data |
|---|---|
| Igntion Temperature of Selected Metals |
|

Key Points
- The combustion of metals is a complex phenomenon influenced by the metal's reactivity with oxygen and its ignition temperature.
- Highly reactive metals like magnesium and sodium can burn with a visible flame at relatively low temperatures.
- Less reactive metals may not burn in the same manner or may require specific conditions to ignite.
- The process of metals burning is more accurately described as oxidation rather than combustion in the traditional sense.
- Understanding the properties of metals and their reactions with oxygen is crucial for applications in materials science, fire safety, and industrial processes.
Advanced Considerations and Applications

Beyond the basic understanding of whether metals can burn, there are advanced considerations and applications of metal combustion in various fields. For example, in aerospace engineering, the combustion characteristics of metals are critical for understanding and mitigating fire risks in aircraft and spacecraft. Similarly, in materials science, researching how metals burn can lead to the development of new materials with tailored properties, such as high-temperature resistance or specific optical properties.
Technical Specifications and Contextual Interpretation
From a technical standpoint, the combustion of metals is often analyzed using thermodynamic and kinetic models. These models help predict the ignition temperatures, burning rates, and products of the combustion reaction. For instance, the combustion of aluminum in air can produce aluminum oxide (Al2O3) as a primary product, which has significant implications for the design of propulsion systems and the safety of industrial processes involving aluminum.
What metals are most likely to burn?
+Highly reactive metals such as magnesium, titanium, sodium, and potassium are more likely to burn due to their strong affinity for oxygen. These metals can ignite and burn at relatively low temperatures compared to less reactive metals.
Is the burning of metals always visible?
+No, not all metal burning is visible. Some metals may glow or smolder without producing a visible flame, depending on the conditions of the reaction and the specific metal involved.
What are the practical applications of understanding metal combustion?
+Understanding metal combustion has practical applications in fire safety, materials science, aerospace engineering, and industrial processes. It helps in the development of new materials, the design of safer systems, and the mitigation of fire risks.
In conclusion, the question of whether metal burns is nuanced and depends on the specific metal and conditions involved. While metals do undergo oxidation reactions that can produce heat and light, the process is distinct from the combustion of organic materials. The study of metal combustion is critical for various technological and industrial applications, highlighting the importance of materials science and chemical engineering in advancing our understanding of these reactions.