5 Tips Protecting Group

Protecting groups are a crucial concept in organic chemistry, allowing chemists to temporarily block or "protect" specific functional groups within a molecule during a chemical reaction. This technique is essential for synthesizing complex molecules, as it enables the selective modification of certain parts of the molecule while leaving others unchanged. In this article, we will delve into the world of protecting groups, exploring their importance, common types, and strategic applications in organic synthesis.
Key Points
- Understanding the role of protecting groups in organic synthesis
- Identifying common protecting groups for various functional groups
- Appreciating the strategic importance of protecting groups in complex molecule synthesis
- Recognizing the challenges and limitations associated with protecting groups
- Exploring innovative approaches to protecting group chemistry
Introduction to Protecting Groups
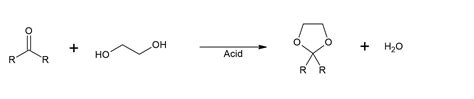
Protecting groups are molecular components that can be attached to and detached from a functional group, effectively “protecting” it from reacting with other reagents during a chemical reaction. This temporary modification allows chemists to control the reactivity of specific parts of the molecule, enabling the synthesis of complex molecules with high precision. The choice of protecting group depends on the type of functional group being protected, the reaction conditions, and the desired outcome of the synthesis.
Common Protecting Groups
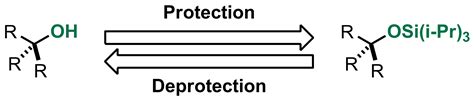
Several protecting groups are commonly used in organic synthesis, each with its own advantages and limitations. For example, tert-butyldimethylsilyl (TBDMS) is a popular protecting group for alcohols, while p-methoxybenzyl (PMB) is often used to protect amines. The selection of a suitable protecting group requires careful consideration of the reaction conditions, as well as the potential for unwanted side reactions or interference with other functional groups in the molecule.
| Functional Group | Common Protecting Group |
|---|---|
| Alcohol | TBDMS, triisopropylsilyl (TIPS) |
| Amine | PMB, benzyloxycarbonyl (Cbz) |
| Carboxylic Acid | ethyl ester, t-butyl ester |
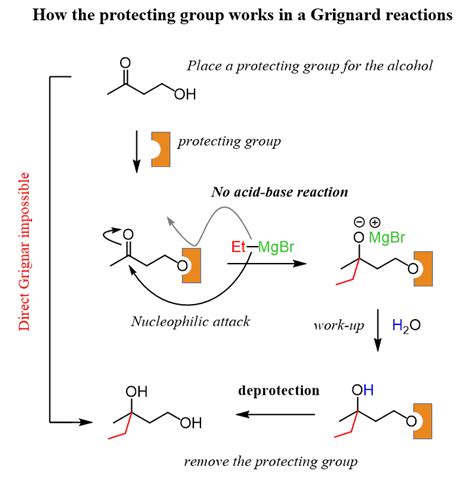
Strategic Applications of Protecting Groups
Protecting groups play a crucial role in the synthesis of complex molecules, such as natural products, pharmaceuticals, and materials. By temporarily blocking specific functional groups, chemists can control the reactivity of the molecule, allowing for the selective formation of bonds and the introduction of functional groups. This strategy is particularly important in the synthesis of molecules with multiple functional groups, where the selective protection and deprotection of specific groups can be used to direct the reaction sequence.
Challenges and Limitations
While protecting groups are a powerful tool in organic synthesis, they also present several challenges and limitations. The choice of protecting group can be difficult, and the protection and deprotection steps can add significant complexity to the reaction sequence. Additionally, the use of protecting groups can lead to unwanted side reactions or interference with other functional groups in the molecule. Furthermore, the removal of protecting groups can be problematic, requiring harsh conditions or specialized reagents.
What is the primary purpose of protecting groups in organic synthesis?
+The primary purpose of protecting groups is to temporarily block or "protect" specific functional groups within a molecule during a chemical reaction, allowing for the selective modification of certain parts of the molecule while leaving others unchanged.
How are protecting groups selected for a particular functional group?
+The selection of a protecting group depends on the type of functional group being protected, the reaction conditions, and the desired outcome of the synthesis. The choice of protecting group requires careful consideration of the potential for unwanted side reactions or interference with other functional groups in the molecule.
In conclusion, protecting groups are a vital component of organic synthesis, enabling the selective modification of complex molecules and the synthesis of high-value compounds. By understanding the role of protecting groups, recognizing common protecting groups, and appreciating the strategic importance of protecting groups in complex molecule synthesis, chemists can unlock the full potential of organic synthesis and push the boundaries of what is possible in the laboratory.
